
Nitrox specialty
Certification card
Medical check
Nitrox and air
Diving equipment for nitrox
Nitrox characteristic
Prevent oxygen poisoning 1
Prevent oxygen poisoning 2
Prevent oxygen poisoning 3
Health management
Dive plans and dive table
|

|
Amount of nitrogen
Nitrox contains less nitrogen than air, which is one advantage over air diving.
At the same dive depth, the amount of nitrogen that dissolves in the body is lower than when breathing air. As a result, the no-decompression limit is longer, and your diving is safer with respect to decompression sickness.
You may also think nitrogen narcosis will be reduced. However, due to the anesthetic effect of high-concentration oxygen gas, Nitrox may cause symptoms similar to nitrogen narcosis.
|
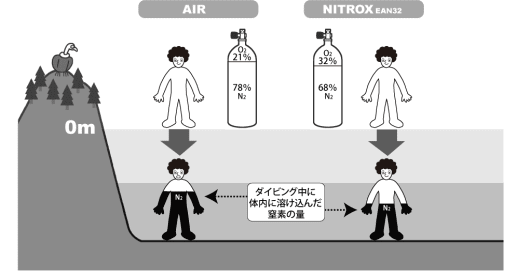
Difference in the amount of nitrogen dissolved in the body |
|
Amount of oxygen
Nitrox contains more oxygen than air, so you can take in more oxygen with each breath when compared at the same breathing volume.
This can make Nitrox feel easier to breathe than air and may reduce breathing gas consumption during the dive.
However, you must pay more attention than in air diving to the risk of oxygen poisoning. |
 |
|
■Oxygen partial pressure
The pressures of oxygen and nitrogen in a breathing gas are called partial pressures.
On land, we breathe air at 1 atm.
In this case, the partial pressure of oxygen in air is 0.21 atm, and the partial pressure of nitrogen is 0.78 atm.
At a depth of 10 m, a diver breathes gas at 2 atm.
When breathing air, the partial pressure of oxygen is 0.42 atm (0.21 atm × 2), and the partial pressure of nitrogen is 1.56 atm (0.78 atm × 2).
On the other hand, EAN32 is Nitrox with an oxygen percentage of 32%. If you breathe it on land, the partial pressure of oxygen is 0.32 atm, and the partial pressure of nitrogen is 0.68 atm.
Also, at a depth of 10 m while breathing EAN32, the partial pressure of oxygen is 0.64 atm, and the partial pressure of nitrogen is 1.36 atm.
Therefore, when using Nitrox, the partial pressure of oxygen in the breathing gas during diving will be higher than when using air.
The oxygen partial pressure can be calculated using the following formula:
Oxygen partial pressure = Nitrox oxygen ratio × (Diving depth ÷ 10 + 1)
For example, when diving to a depth of 20 m using EAN32, the oxygen ratio of EAN32 is 0.32. Using the formula above, you can calculate the oxygen partial pressure as follows:
Oxygen partial pressure = 0.32 × (20 ÷ 10 + 1) = 0.96
|
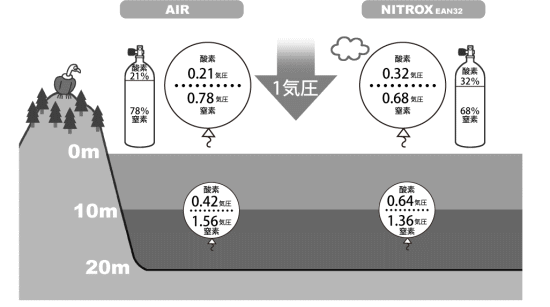 Difference of breathing gas oxygen concentration |
|
■Oxygen poisoning
The oxygen poisoning problem during recreational diving is central nervous system oxygen poisoning.
Avoid deep dives and long dives where oxygen absorption in the body exceeds the specified level. Also avoid dives with intense exercise, where carbon dioxide tends to accumulate in the body, and dives in low water temperatures. |
Intense exercise is not recommended |
|
Also, if the regulator has high breathing resistance and it is difficult to breathe, or if you breathe shallow and fast, ventilation may be insufficient, increasing the risk of oxygen poisoning.
Oxygen poisoning can cause convulsions, and you may no longer be able to hold the regulator in your mouth.
Symptoms such as dizziness, visual impairment (narrowing of the field of view), tingling and tightness of the lips or eyelids, nausea, and headaches are considered signs of oxygen poisoning.
Please stop the dive immediately. |
 |

Beware of dizziness and headaches |
|
|
|
  |
|

